Supercritical fluid
Critical point occurs under conditions such as specific values of pressure and temperature at which no phase boundaries exist. Therefore, the supercritical fluid is any substance at a temperature and pressure above its critical point. |
|
 |
| |
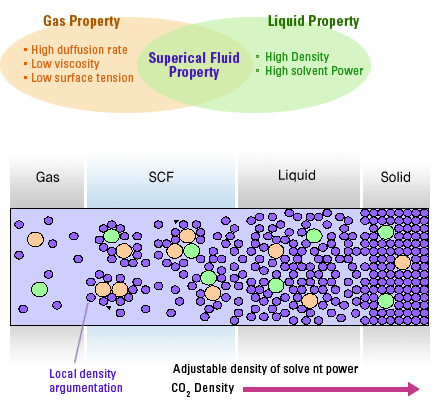
 Physical character of Supercritical Physical character of Supercritical |
• It has properties between those of a gas and a liquid.
• Properties can be changed by the pressure and temperature of the fluid.
• It is relatively rapid compared with liquid because of the low viscosities and high diffusivities associated with
supercritical fluids. |
| Phase |
Density (Kg/㎥) |
Viscosity,(CP) |
Diffusion x 108(㎡/sec) |
| Gas |
1.0 |
0.05-0.35 |
100-10000 |
| SCF |
200-900 |
0.2-1.0 |
0.1-0.3 |
| Liquid |
800-1000 |
3.0-20 |
0.05-0.2 |
|
 |
 Critical point of fluid state Critical point of fluid state |
| Solvent |
Critical Temp.
(℃) |
Critical Pressure
(bar) |
Density
(g/㎤) |
Molecular Weight
(g/mol) |
| Carbon dioxide (CO2) |
31.1 |
73.8 |
0.469 |
44.01 |
| Water (H2O) |
373.1 |
220.5 |
0.348
|
18.02 |
| Methane (CH4) |
-87.75 |
46 |
0.162 |
16.04 |
| Ethane (C2H6) |
32.4 |
48.8 |
0.203 |
30.07 |
| Propane (C3H8) |
96.8 |
42.5 |
0.217 |
44.09 |
| Ethylene (C2H4) |
9.4 |
50.4 |
0.215 |
28.05 |
| Propylene(C2H2) |
91.75 |
46 |
0.232 |
42.08 |
| Methanol (CH3OH) |
239.45 |
80.9 |
0.272 |
32.04 |
| Ethanol (C2H5OH) |
240.9 |
61.4 |
0.276 |
46.07 |
| Acetone (C3H6O) |
235.1 |
47.0 |
0.278 |
58.08 |
|
| Carbon dioxide and water are commonly used for supercritical fluids |
| |
 Ideal supercritical fluid Ideal supercritical fluid |
• No corrosion & safety
• Low temperature & pressure for critical point.
• High solubility
• Easy to buy and cheaper price.
• Harmlessness |
| |
 Feature of supercritical fluid Feature of supercritical fluid |
• Eco-friendly clean technology to environment and human
• High technology for high purity and high quality(extraction system)
• Technology of saving energy.
• No waste of solvent(fluid) |
| |
 Character of supercritical fluids Character of supercritical fluids |
| 1) Character of Supercritical CO2 |
• Nontoxic and nonflammable, rare properties.
• Eco-friendly fluid (recycle system)
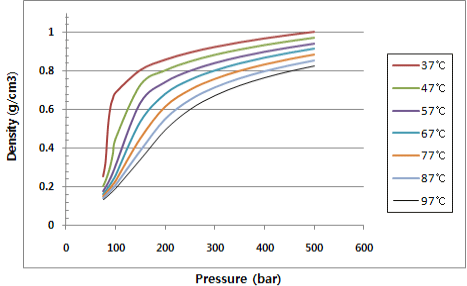
• Low critical point of temperature and pressure(31.0 6degree C, 74 bar)
• High diffusion coefficient(compared with liquid)
*Density change by temperature and pressure |
 |
Supercritical CO2 system has been the focus of new technology.
Especially, its application are expanding various industry which are food industry, chemical industry, raw-material industry, environmental industry, energy industry; that need environmental conservation and to manufacture product with high quality. |
| |
| 2) Character of Supercritical Water |
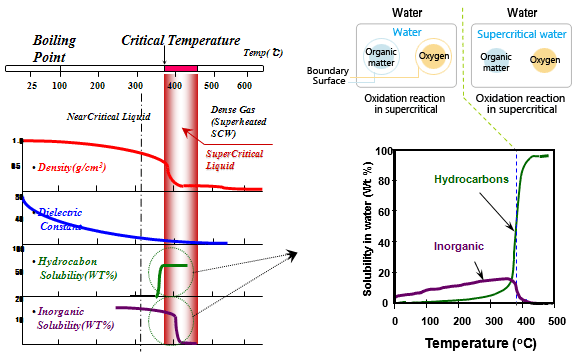
• Chemical properties(density, dielectric constant, conductivity, solvency) can be changed above critical point(374 degree C, 221 bar)
• Non-polar solvent
: Dielectric constant of supercritical water has quasivalue with non-polar solvent of benzene, ethyl ether, hexane)
• Supercritical water is excellent solvent to organic matter that is difficult to be dissolved by water.
• It is possible for complete oxidation due to high oxygen and there are no pollutants after process.
• Reaction velocity is rapid by single phase oxidation reaction. |
 |
| |
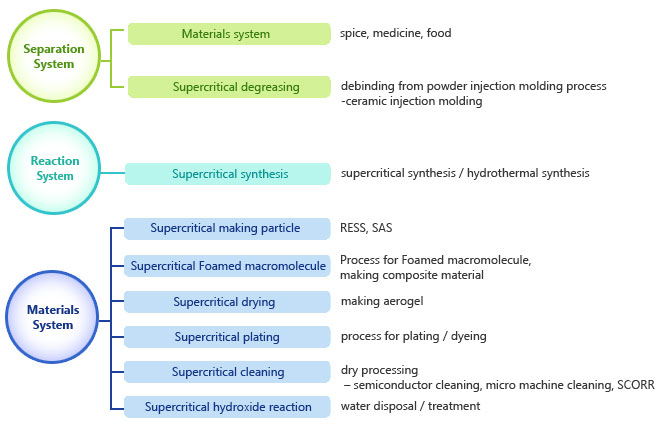
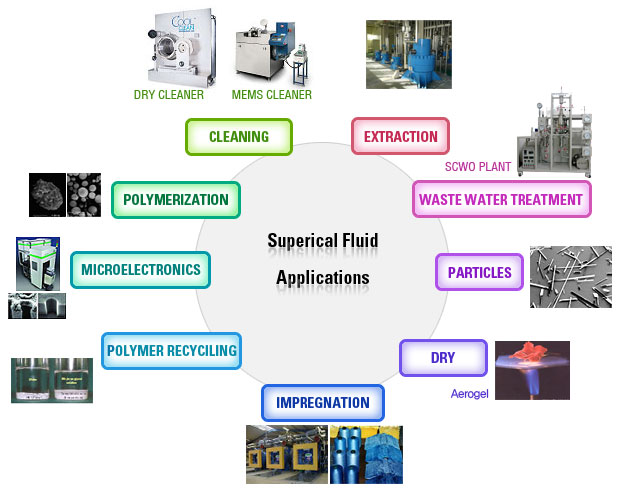
 Application according to system Application according to system |
 |
| |
 Application Application |
 |